

Yes, it is true, hydrogen bonding (N-H bonds makes between molecules) and dipole dipole interaction (interaction between two dipole) and london dispersion forces occur between nh3 molecules. Then the only interaction between them will be the weak London dispersion (induced dipole) force If the molecules have no dipole moment, (e.g., H2, noble gases etc.) Does Cl2 have London dispersion forces?ģ) F2, Cl2, Br2 and I2 are non-polar molecules, therefore they have London dispersion forces between molecules. Noble gases exhibit only London dispersion forces. Which substances exhibit only London dispersion forces? If you see any of those cases, then that will help you identify that it’s London Dispersion Force These are the three types of intermolecular forces London Dispersion Forces which are the weakest, which occur between nonpolar noble gases and same charges.
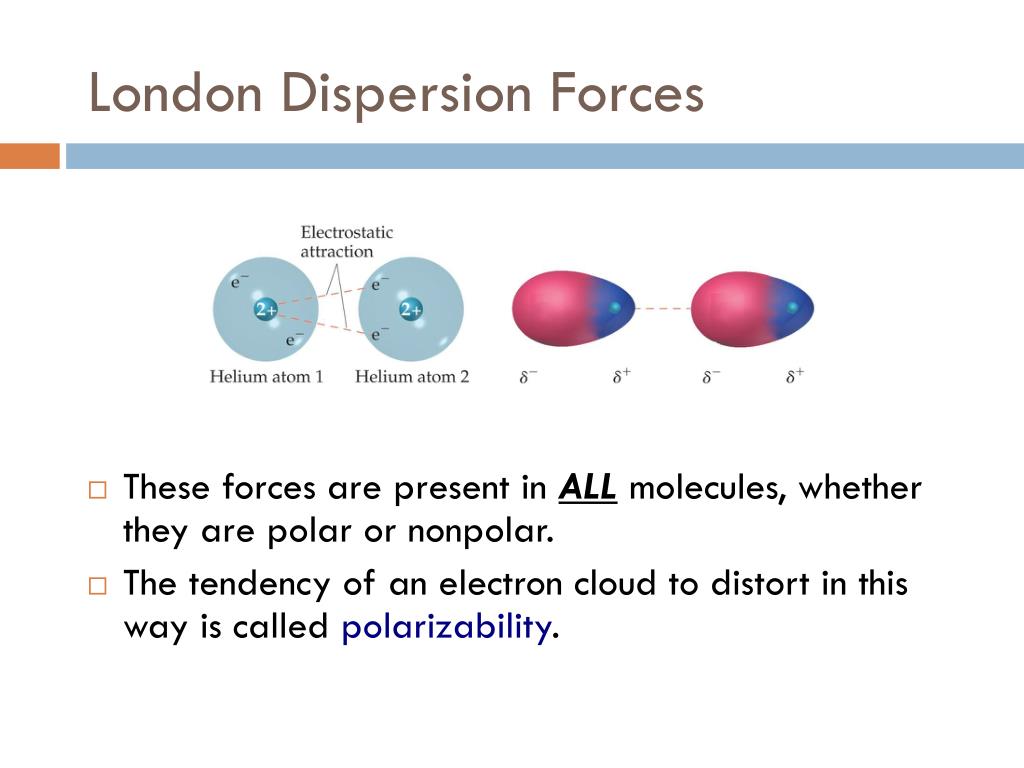
How do you know if a molecule has London dispersion forces? London dispersion forces are part of the van der Waals forces, or weak intermolecular attractions. These London dispersion forces are often found in the halogens (e.g., F 2 and I 2), the noble gases (e.g., Ne and Ar), and in other non-polar molecules, such as carbon dioxide and methane. What type of molecules have London dispersion? Is CH4 polar or nonpolar?Ĭarbon dioxide is a linear and non-polar molecule so the only intermolecular force present in CO2 is London dispersion forces or Van der Walls forces.
DOES HI HAVE A SINGLE LONDON DISPERSIO FORCE FULL
This is because hydrogen bonds are a type of electrostatic interaction, which is only possible in molecules in which… See full answer below. London Dispersion Forces & Temporary Dipole – Induced Dipole Interactions – Intermolecular ForcesĢ7.0 similar questions has been found Is CH4 hydrogen bonding?Īnswer and Explanation: CH4 cannot form hydrogen bonds. Is CH4 dispersion or dipole-dipole? Does CF4 have London dispersion forces? Molecules can have any mix of these three kinds of intermolecular forces, but all substances at least have LDF.

There are three types of intermolecular forces: London dispersion forces (LDF), dipole- dipole interactions, and hydrogen bonding. Only polar molecules will show dipole-dipole interactions, and all will exhibit london-dispersion forces. Therefore the strongest intermolecular forces between CH4 molecules are Van der Waals forces. What type of intermolecular forces dominate in CH4?Īlso CH4 molecules cannot have permenant dipole-dipole attractions because each of the species bonded to the carbon is identical and CH4 has a tetrahedral shape. Because methane is a non-polar molecule it is not capable of hydrogen bonding or dipole-dipole intermolecular forces.


 0 kommentar(er)
0 kommentar(er)
